What is being done to fight antimicrobial resistance?
Last Updated : 21 November 2023Improper and excessive use of antimicrobials in both human health care and agricultural production domains has accelerated the development of bacterial defences against them and the rise of antimicrobial resistance (AMR). This article explores what European and international organizations are doing to tackle this issue.
Before delving into the topic, it’s useful to briefly recap what an antimicrobial and antimicrobial resistance are:
- An antimicrobial is a general term used for any drug (e.g., antibiotics, antivirals, antifungals and antiparasitics) that works to treat and prevent infections caused by microorganisms, such as viruses, bacteria, fungi and parasites, by killing or inhibiting their growth.
- Antimicrobial resistance is the resistance of any microorganism to an antimicrobial drug that was initially effective in treating infections caused by it.1
What is Europe doing against antimicrobial resistance?
Surveillance
The European Food Safety Authority (EFSA) works closely with other EU agencies to tackle AMR in Europe such as the European Centre for Disease Prevention and Control (ECDC) and the European Medicines Agency (EMA). Monitoring and reporting of AMR in zoonotic bacteria in humans, animals and in food are collected annually by the EU Member States and reporting countries, analysed and presented in a yearly EU Summary Report.
Legislation
Several legislative measures have been taken in Europe to enhance the regulation and control of antimicrobials in primary food production. In June 2023 the Council of the European Union adopted the Recommendation to strengthen EU action against antimicrobial resistance.2,3 With regard to food production, the Council encourages Member States:
- to improve the health and welfare of food-producing animals to decrease the occurrence and spread of infectious diseases in farming and subsequently reduce the need for antimicrobial use.
- to reduce the overall EU sales of antimicrobials used for farmed animals and in aquaculture by 50% by 2030.
- to foster the development and placing on the market of effective and evidence-based alternatives to the use of antimicrobials and of vaccines for animal health.
Research and innovation
Since 2014, the EU’s research and innovation funding programmes, Horizon 2020 (2014-2020) and Horizon Europe (2021-2027) have mobilised over €690 million to support research and innovation on AMR as part of a broader research portfolio on infectious diseases. The research is both focused on the development of new antimicrobials and on new ways to face the spread of AMR.3 Moreover, other health and research programmes provide important funding to combat AMR. Among these, the EU4Health programme (adopted as a response to the COVID-19 pandemic and to reinforce crisis preparedness in the EU) has set aside €50 million for joint action on AMR.
In the first 2 years of Horizon Europe, €32.5 million has been committed to 13 research projects related to tackling antimicrobial resistance. EUFIC was involved in two of them:
- In HOLiFOOD4, researchers are developing tools and monitoring systems using technologies such as artificial intelligence (AI) and Big Data, which will help to early identify emerging risks of a microbiological nature (like AMR) or chemical nature across three studied supply chains (poultry, maize, lentils) and improve monitoring.
- Within CIRCLES5 researchers are designing and validating microbial communities or microbiome-tailored actions to reduce antimicrobial resistance (AMR) and enhance the safety and recycling potential of plants, poultry, pigs, aquaculture and marine fish productions.
What is being done in the world against antimicrobial resistance?
Globally, the fight against AMR follows the “One Health” approach. This concept has been recognized since the 1800s, even though human and animal medicine were practised separately until the 20th century. Nowadays, the term is used to describe a principle which recognises that human and animal health are interconnected and linked to the environment, that diseases are transmitted from humans to animals and vice versa and must therefore be tackled in both.6
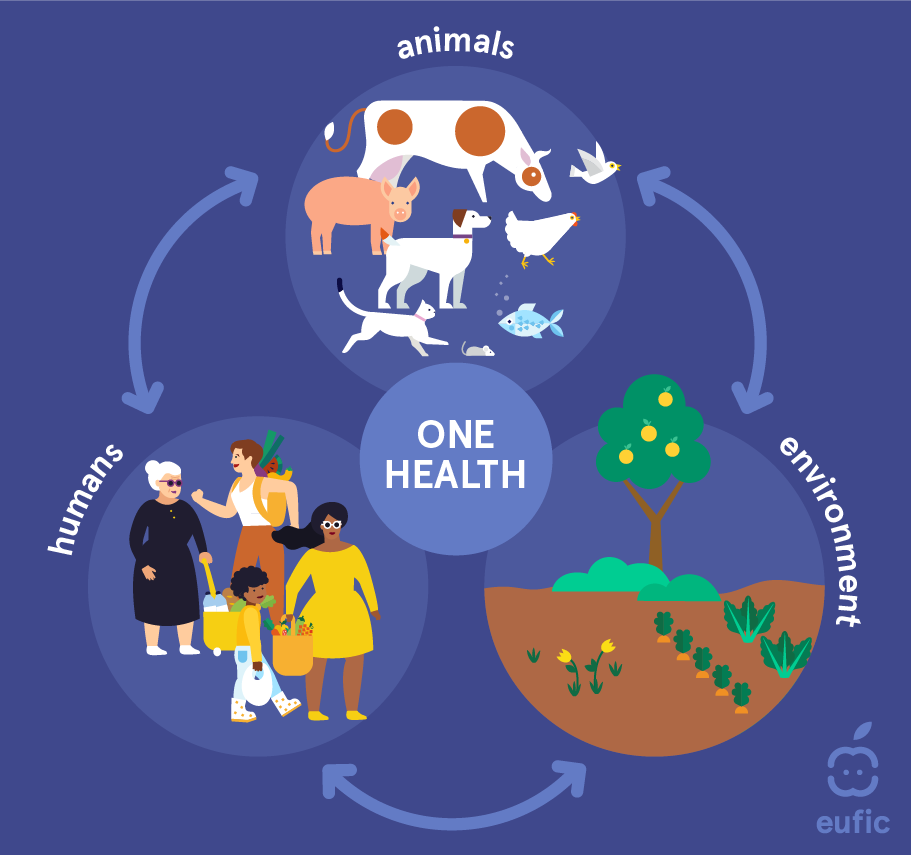
In 2015, the Sixty-eighth World Health Assembly endorsed a global action plan7 to address the issue of resistance to antibiotics and other antimicrobial medicines. The following year, Heads of State made a political declaration during the United Nations General Assembly, highlighting the commitment to a coordinated approach in addressing antimicrobial resistance across various sectors, including human health, animal health, and agriculture. Since then, the World Health Organization (WHO) has been supporting Member States in developing national action plans on antimicrobial resistance, in line with the five strategic objectives of the global action plan:
- To improve awareness and understanding of antimicrobial resistance.
- To strengthen surveillance and research.
- To reduce the incidence of infection.
- To optimize the use of antimicrobial medicines.
- To ensure sustainable investment in countering antimicrobial resistance.
In parallel, WHO has been leading (or co-leading) multiple initiatives to address antimicrobial resistance at every level. Among these, there are:
- The World Antimicrobial Awareness Week (18-24 November), which aims to increase awareness of antimicrobial resistance worldwide and to encourage best practices. The theme for 2023 is “Preventing antimicrobial resistance together”, to highlight that AMR is a threat to humans, animals, plants and the environment.
- The Global Antimicrobial Resistance Surveillance System, which supports a standardized approach to the collection, analysis and sharing of data related to antimicrobial resistance, including AMR in the food chain, to inform decision-making and drive local, national, and regional action.
Summary
The well-being of humans and animals are intricately intertwined. Some pathogens can affect both human and animal communities. Subsequently, some animals could act as “vectors” that disseminate AMR to humans, for example through direct contact or human food contamination. Consequently, adopting the "One Health" strategy becomes indispensable in effectively managing and averting such diseases across both animal and human populations. By ensuring the protection of animal health and preventing the outbreak of animal-related illnesses, we can safeguard public health, ensure food security, sustain rural economies, and preserve the environment.
 This article was produced in collaboration with HOLiFOOD. HOLiFOOD has received funding from the European Union’s Horizon 2020 Research and Innovation programme under Grant Agreement No. 101059813.
This article was produced in collaboration with HOLiFOOD. HOLiFOOD has received funding from the European Union’s Horizon 2020 Research and Innovation programme under Grant Agreement No. 101059813.
References
- EUFIC. Antimicrobial Resistance (Q&A). Retrieved from
- European Commission (2023). Council Recommendation on stepping up EU actions to combat antimicrobial resistance in a One Health approach. Accessed 10 November 2023
- European Commission (2023). Frequently Asked Questions: Recommendation on stepping up EU actions to combat AMR in a One Health approach. Accessed 10 November 2023
- Holifood project. Accessed 10 November 2023
- Circles project. Accessed 10 November 2023
- European Commission (2017). A European One Health Action Plan against Antimicrobial Resistance (AMR). Accessed 10 November 2023
- World Health Organisation (2020). Antibiotic resistance. Accessed 10 November 2023
