eufic
food facts for
healthy choices
what we do
We create science-based content to empower and facilitate the adoption of healthier and more sustainable diets and lifestyles among European citizens, with a focus on four main goals:
our latest articles
Stay up-to-date with our most recent content! Dive into new topics, gain insight into the latest evidence, and discover helpful tips.
newsroom
Learn about our latest initiatives and take a look at our Food Facts summaries addressing misrepresented information about food and health in the media.
we are involved in
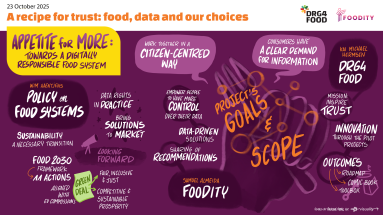
Advancing the European conversation on innovation and sustainability in the food sector. Discover the european projects we're involved in:

resources
Tap into a rich array of resources (e.g., infographics, webinars, guides, etc.), on a variety of food and health-related topics! read more